by Debra Wilson
How can you decorate a mineral for Christmas? If it’s the right mineral, all you need to do is shine a SWUV (short wave ultraviolet) light on it. Such is the case with a mineral that is known as “Christmas Ore.” Under normal light it looks like kind of a drab rock as the one in this photo does.

This specimen of calcite (tan color), willemite (brown color) and franklinite (black color) is on display in Hillman Hall of Minerals and Gems in the Fluorescence & Phosphorescence exhibit and was donated by the Sterling Hill Mining Museum for this exhibit. It originated from the Sterling Hill mine in Ogdensburg, in the Franklin Mining District of New Jersey. When you shine the SWUV light on it you will see why it is called “Christmas Ore” because it glows with the colors of Christmas. The calcite glows a bright red and the willemite glows a bright green, as you can see in this photo.
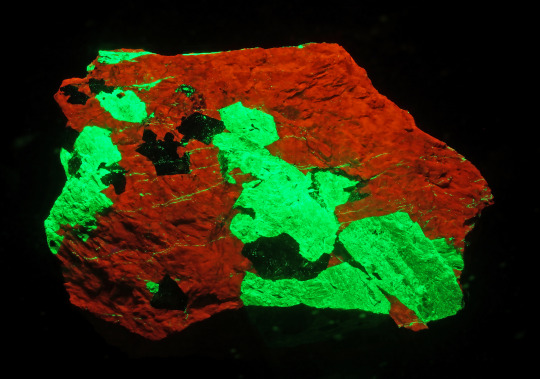
This glowing is known as fluorescence and the Franklin Mining District is known as the fluorescent capital of the world. The Franklin and Sterling Hill ore bodies are the source of at least 350 mineral species.At present, over 80 fluorescent mineral species are known from the area. Willemite and calcite are the most common fluorescents in these ore bodies and are known as “Christmas Ore” when they occur together in the same specimen.
So, what causes the fluorescence? Fluorescence usually occurs when specific impurities known as “activators” are present within the mineral. These activators are typically cat ions of metals such as: tungsten, molybdenum, lead, boron, titanium, manganese, uranium and chromium. Rare earth elements such as europium, terbium, dysprosium, and yttrium are also known to contribute to the fluorescence phenomenon. Fluorescence can also be caused by crystal structural defects or organic impurities. Calcite (CaCO3) and willemite (Zn2SiO4) are examples of minerals that in their pure state do not fluoresce but add a little divalent manganese (Mn2+) and they will fluoresce red and green, respectively.
There are two other specimens of calcite and willemite, also from the Franklin District, in the Fluorescence & Phosphorescence exhibit, shown here under normal light and under SWUV light.




Come to Hillman Hall of Minerals and Gems to hear a more detailed explanation of the phenomenon of fluorescence and see all 21 specimens in the exhibit from world-wide localities that glow under the ultraviolet lights.
Debra Wilson is the Collection Manager for the Section of Minerals at Carnegie Museum of Natural History. Museum employees are encouraged to blog about their unique experiences and knowledge gained from working at the museum.
Related Content
Thanksgiving and Nutritional Mineralogy
Ask a Scientist: Why do some minerals glow?
Carnegie Museum of Natural History Blog Citation Information
Blog author: Wilson, DebraPublication date: December 13, 2018
