This event has ended. Keep an eye on our website and social media for more fun events!
minerals and gems
New Mineral Acquisitions
I had a successful trip to Tucson, Arizona in January/February of this year. This is an annual event where the Section of Minerals participates in the Tucson Gem & Mineral Show by not only exhibiting a mineral display, the Carnegie Mineralogical Award is also presented during the show, and many of the minerals acquired for the collection are found amongst the dozens of venues around the city where vendors are selling their specimens. Numerous motels turned into shopping centers starting as early as January 28th, where each room is a separate store for an individual vendor. Tent shows were set up along streets and in parking lots. The Tucson Convention Center housed two major shows: The American Gem Trade Association Show (AGTA), which was held February 5th through 10th, and the Tucson Gem & Mineral Show, which was held February 14th through 17th. In total, I brought back 10 specimens acquired for the collection, five of which were acquired specifically for exhibit in Hillman Hall of Minerals and Gems or Wertz Gallery: Gems and Jewelry. Two gemstones were put on display in the “What is a Gemstone?” exhibit in Wertz Gallery on March 12th: a 44.27 carat, cushion cut spodumene from Afghanistan, and a 7.08 carat, trillion cut titanite (also known as sphene) from Zimbabwe.


A special exhibit to highlight the museums acquisitions will be put in Hillman Hall on March 26th that will feature a world class Kermesite specimen from China. This specimen measures 20 cm and is probably the finest example of its species in the world. The largest kermesite in our collection prior to this acquisition is only 3cm.

Two other specimens will be going on display soon in the Systematic Collection area of Hillman Hall: a blue tabular beryl from Afghanistan in the Silicates 2 case, and a bornite from Montana in the Sulfides 2 case. The beryl is a recent discovery in Afghanistan that is different than any other type of beryl, while the bornite was collected sometime in the 1950s in Butte, Montana which is known as the best locality in the United States for this species. Watch for announcements of when these two special pieces go on exhibit!


Debra Wilson is the Collection Manager for the Section of Minerals at Carnegie Museum of Natural History. Museum employees are encouraged to blog about their unique experiences and knowledge gained from working at the museum.
A Perfect Mineral for the Christmas Season
by Debra Wilson
How can you decorate a mineral for Christmas? If it’s the right mineral, all you need to do is shine a SWUV (short wave ultraviolet) light on it. Such is the case with a mineral that is known as “Christmas Ore.” Under normal light it looks like kind of a drab rock as the one in this photo does.

This specimen of calcite (tan color), willemite (brown color) and franklinite (black color) is on display in Hillman Hall of Minerals and Gems in the Fluorescence & Phosphorescence exhibit and was donated by the Sterling Hill Mining Museum for this exhibit. It originated from the Sterling Hill mine in Ogdensburg, in the Franklin Mining District of New Jersey. When you shine the SWUV light on it you will see why it is called “Christmas Ore” because it glows with the colors of Christmas. The calcite glows a bright red and the willemite glows a bright green, as you can see in this photo.
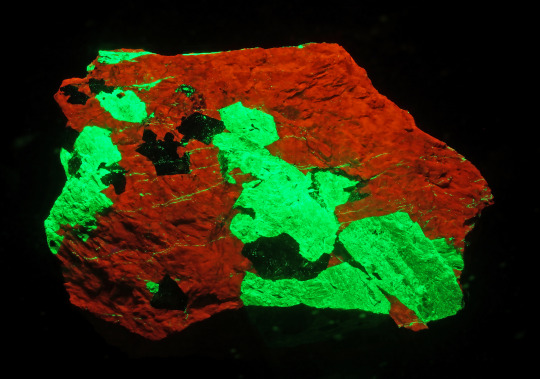
This glowing is known as fluorescence and the Franklin Mining District is known as the fluorescent capital of the world. The Franklin and Sterling Hill ore bodies are the source of at least 350 mineral species.At present, over 80 fluorescent mineral species are known from the area. Willemite and calcite are the most common fluorescents in these ore bodies and are known as “Christmas Ore” when they occur together in the same specimen.
So, what causes the fluorescence? Fluorescence usually occurs when specific impurities known as “activators” are present within the mineral. These activators are typically cat ions of metals such as: tungsten, molybdenum, lead, boron, titanium, manganese, uranium and chromium. Rare earth elements such as europium, terbium, dysprosium, and yttrium are also known to contribute to the fluorescence phenomenon. Fluorescence can also be caused by crystal structural defects or organic impurities. Calcite (CaCO3) and willemite (Zn2SiO4) are examples of minerals that in their pure state do not fluoresce but add a little divalent manganese (Mn2+) and they will fluoresce red and green, respectively.
There are two other specimens of calcite and willemite, also from the Franklin District, in the Fluorescence & Phosphorescence exhibit, shown here under normal light and under SWUV light.




Come to Hillman Hall of Minerals and Gems to hear a more detailed explanation of the phenomenon of fluorescence and see all 21 specimens in the exhibit from world-wide localities that glow under the ultraviolet lights.
Debra Wilson is the Collection Manager for the Section of Minerals at Carnegie Museum of Natural History. Museum employees are encouraged to blog about their unique experiences and knowledge gained from working at the museum.
Related Content
Thanksgiving and Nutritional Mineralogy
Ask a Scientist: Why do some minerals glow?
Carnegie Museum of Natural History Blog Citation Information
Blog author: Wilson, DebraPublication date: December 13, 2018
Share this post!
Born to the Purple
by Debra Wilson

What comes to mind when you hear the phrase “born to the purple”? Most people will probably think of royalty.
The color purple has been associated with royalty since ancient times, in large part because the murex shellfish-based Tyrian purple dye (aka Royal purple or Imperial purple), produced by the Phoenician city of Tyre during the Bronze Age, was very expensive to make and thus only the wealthiest classes, including the nobility, could afford it. Its striking color and resistance to fading made clothing dyed with Tyrian purple highly desirable and the ancient Romans adopted purple as a symbol of imperial authority and status. The togas of the Senators were trimmed in purple and a completely purple toga was worn by the person occupying the powerful office of Censor. It was the Censor’s job to determine which Senators were still worthy of office and who should and should not be on the roster of Rome’s leading citizens.
The color purple was not only reserved as a status symbol for clothing but was also used in Roman monuments and buildings. “Imperial Porphyry” is an igneous rock that contains hematite and the manganese-bearing mineral piemontite that makes it similar in color to the Tyrian purple dye. Porphyry (from the Greek meaning purple) has a hardness of 7 out of 10 on the Mohs hardness scale, comparable to steel or quartz, which made it very suitable for carving. It took very strong, well-tempered steel to cut it, and was very challenging to achieve any great degree of precision in the cutting. The Romans developed steel good enough for the task, but the process was lost in the Middle Ages, making Roman porphyry artifacts not only symbols of the Caesars but also of Rome’s great technological achievements.
The Imperial Porphyry was also rare and expensive because it came from only one quarry discovered in 18AD by the Roman Legionnaire Caius Cominius in the far away Eastern Desert of Egypt, located near the Red Sea at a place that became known as Mons Porphyrites. Can you imagine extracting huge blocks of heavy porphyry and then transporting it by ship from Egypt to Rome in ancient times? The rock was imported in large quantities, most actively during the times of Nero, Trajan and Hadrian, to both Rome and Constantinople and was used in their statues, monuments, columns, and sarcophagi. One free-standing pavilion in the Great Palace of Constantinople was completely clad in the purple Imperial Porphyry and this chamber was where the empress would give birth. By now you might have guessed that the phrase “Born to the Purple” was referring to the purple clad porphyry chamber where the princes and princesses were born.

A wonderful example of an Imperial Porphyry carving is that of the Four Tetrarchs (circa 305 AD) pictured in the above photo. Originally it was thought to reside in a public square in Constantinople but was moved to a corner of the facade of St. Mark’s Basilica in Venice, Italy sometime in the Middle Ages, perhaps around 1204 AD. This sort of thing happened quite often because the Imperial Porphyry was so highly prized, and the locality of the Egyptian quarry had been lost around the 4th century AD and was not rediscovered until 1823. So the only source of this treasured porphyry in the interim came from things the Romans had built. It seems that every prince or republic or sculptor of the time who wanted this status symbol of Roman power would scavenge it from some old Roman temple, column, or sarcophagus. Much of the Imperial Porphyry seen around Europe today has been “repurposed” and can be found from Britain to Kiev.

Pictured above is a fragment of polished Imperial Porphyry (CM17465) from the ruins of Rome in the Carnegie Museum of Natural History’s Mineral collection, measuring 24.6 x 17.5 x 2.6 cm. It was acquired from Wards National Science Establishment in 1897. The white speckles you see embedded in the rock are plagioclase feldspar known as phenocrysts, which can range in color from white to pink.
Debra Wilson is the Collection Manager for the Section of Minerals at Carnegie Museum of Natural History. Museum employees are encouraged to blog about their unique experiences and knowledge gained from working at the museum.
Fred the Crystal Skull
by Debra Wilson
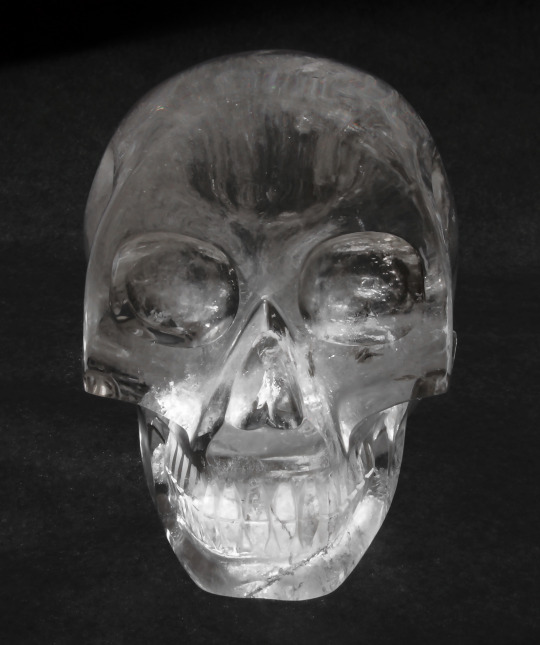
Just about every year since the Carnegie Museum of Natural History acquired it, Fred the Crystal Skull has made an appearance in Hillman Hall of Minerals and Gems right around Halloween. So how did we acquire a crystal skull and how did it get the name Fred you ask? Just to set the record straight right off the bat, Fred is not one of the dozen or so mysterious skulls that some think were carved by an ancient Mesoamerican civilization thousands of years ago. Our skull was carved and polished from a single quartz crystal with modern tools in Brazil and was donated to the museum in 2004 by South American Gems, Ltd located in Guarapari, Espirito Santo, Brazil.Germany, China and Brazil currently produce thousands of carved crystal skulls every year in numerous sizes. Fred measures 7.8 inches high by 5 inches wide, which is slightly smaller than the average human skull (8 to 9 inches high and 6 to 7 inches wide) so he was named after a man of small stature, namely the step father of the former Head of the Section of Minerals Marc Wilson. Marc was Section Head from August 1992 to August 2017.

As you can see in the photograph of Fred, he has some internal flaws and fractures which is very common in the mineral quartz. Chemical impurities, physical flaws and twinning in natural quartz are issues that caused industry to develop a commercial process of manufacturing pure, electronics-grade quartz that can be used in circuits for consumer products such as televisions, radios, computers, cell phones and electronic games, just to name a few, and for crystal-controlled clocks and watches. As it so happens, the Section of Minerals also has a few lab-grown quartz crystals in the collection, including a large crystal nicknamed The Football that is nearly a foot across.

You will notice it is so clear that you can see the growth patterns of the bottom surface through the crystal. The Football was part of a donation of 57 lab created specimens given to the Section of Minerals in 2017 by Lynn Boatner just before he retired from Oak Ridge National Laboratory in Tennessee.
Debra Wilson is the Collection Manager for the Section of Minerals at Carnegie Museum of Natural History. Museum employees are encouraged to blog about their unique experiences and knowledge gained from working at the museum.
Related Content
Beauty from the Ashes
by Debra Wilson

When Mount St. Helens erupted in the State of Washington on May 18, 1980, it became the deadliest and most economically destructive volcanic eruption in the history of the contiguous United States. The devastating results were not only measured by the fatalities and massive destruction but it also left behind about 540,000,000 tons of ash over an area of more than 22,000 square miles. The enormous task of cleanup was daunting. This is where serendipity stepped in to create great beauty from the ashes.
During the salvage effort, workers from a regional timber company were using acetylene torches to cut through twisted metal debris and they accidentally discovered that the torch melted the volcanic ash into a green glassy substance. This led to laboratory experiments that determined green glass could be produced by heating the ash to 2700° Fahrenheit and then rapidly cooling it. The glass quickly began being commercially produced and faceted into gemstones. It is marketed under the names Obsidianite, Helenite, Emerald Obsidianite or Mount St. Helens Obsidian. Its stunning green color has made it an attractive alternative to the more expensive emerald gemstone, though not as durable (a hardness of 5 to 5 ½ as compared to 7 ½ to 8 for emerald). Blue and red varieties are also produced by adding coloring agents to the melt.
The Section of Minerals obtained a faceted stone of Obsidianite as part of a donation of gemstones in 2009. It is a green oval cut stone, as you can see from the photo, and weighs 42.1 carats. This stone is now on display in the Treated & Synthetic Stones case in Wertz Gallery.
Debra Wilson is the Collection Manager for the Section of Minerals at Carnegie Museum of Natural History. Museum employees are encouraged to blog about their unique experiences and knowledge gained from working at the museum.
